
The U.S. Department of Defense signed a contract with a U.S. company that will boost capacity of nitrile glove production in the U.S. by 2.31 billion gloves per year by May 2023.

The U.S. Department of Defense signed a contract with a U.S. company that will boost capacity of nitrile glove production in the U.S. by 2.31 billion gloves per year by May 2023.
Nurses need a seat at the table when discussing PPE stockpiling and purchasing practices to share their lived experiences and help the team discover where practice deviated from plans. Infection preventionists should support the nurses in these discussions as allies.

Individuals vaccinated against COVID-19 do not need to wear a mask outdoors when in small groups, when dining outside, or when biking or running, the CDC announced. However, face-covering precautions should still be taken in some settings.

Surgical masks offer better protection against aerosolized particles than face shields or even better than face shields and surgical masks used together, study finds.

Beau Wangtrakuldee, PhD: “In the health care industry in general, small sizes are typically based on Caucasian males, so once you get to women who truly have smaller frames there are no products available for them.”

The shape and material composition of the N95 respirators varied widely from manufacturer to manufacturer, which can result in variations in the efficacy of a particular method from one product to the next.

Hospitalizations for COVID-19 dropped by a statistically significant 5.5 percentage points for adults from 18 to 64, compared to the hospitalization rates in the 4 weeks preceding the implementation of the mask mandates.

Double-masking can prove more cumbersome for many. If the goal is to protect the mask underneath, perhaps consider a face shield or a strategy for cleaning the mask more frequently.

Infection preventionists have the skill set to provide guidance beyond the health care setting. We know how to select PPE and how to use it. Cleaning and disinfection are like breathing to us.

2021 will likely mean a mixture of things for infection preventionists (IPs). First, a focused effort on vaccine education. While this is a larger effort, IPs have always played a significant role in education and answering questions while rounding on the units and clinics.
Kristy Warren: “We need to do everything we can to help protect our providers when performing these aerosol generating procedures. And subsequently those providers that enter the room or exit the room after these procedures have occurred.”
Though tough months lie ahead for infection preventionists and other healthcare professionals, hope remains that at some point in 2021 things will begin to settle down. In the end, it comes down to a simple formula: We win, COVID-19 loses.
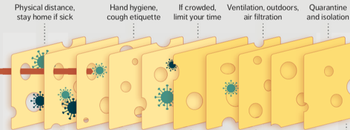
In essence, infection prevention and control isn’t just one measure, like personal protective equipment (PPE), but all of these layers. Each layer is imperfect but plays a critical role in reducing risk.

Compare transmission data for patients on contact precautions using the recommended full complement of PPE versus transmissions for patients on contact precautions when PPE was being utilized differently or not at all.
When an 850-bed urban hospital fought off COVID-19 in part by having to relax infection prevention protocols, the opportunistic and deadly carbapenem-resistant Acinetobacter baumannii (CRAB) struck.
CRAB has chameleon-like tendencies that allow it to absorb material from other organisms, and that allows it to ward off most antibiotics. It’s a Superbug.

Faced with greater than three times the number of cases as the last surge, along with exponential growth with no end in sight, there is little hope healthcare workers can safely treat patients without a drastic change in policy and a more productive and secure supply line.

Ashish Diwanji: “The personal protective equipment made and sold in the US has to abide by the standards set up by NIOSH …. The PPE made and sold from China do adhere to the Chinese standards, but their standards are different than ours.”
“[T]here is a need for early education to enforce correct PPE use to alleviate personal risk concerns. This includes re-education of the donning and doffing of PPE to confirm staff are effectively protecting themselves. Data suggests substantial self-contamination risk occurs when doffing PPE….”

Sharon Ward-Fore, MS, MT(ASCP), CIC: “I’m hoping that healthcare facilities will find the value in their infection preventionists and understand how important a role they play as far as training on PPE and disinfectants, and in hand hygiene, being kind of a boots on the ground people on the floor to see things firsthand.”

When healthcare workers using the red box stepped into the patients’ rooms, there was “significantly increased non-compliance” with PPE and hand hygiene protocols compared to those healthcare workers who went into rooms without red boxes.

Perhaps now is the time that innovation begins to rely more heavily on infection preventionists and our valuable insight into the world of healthcare PPE. The changes we help guide now, can help make healthcare safer and infection prevention easier.
When COVID-19 struck, the proper use of PPE and greater attention to hand hygiene and cleaning surfaces became the norm. When that happened, rates of Clostridium difficile decreased significantly.

Peter Walter, PhD: “We think of AeroNabs as a molecular form of PPE that could serve as an important stopgap until vaccines provide a more permanent solution to COVID-19.”
Sharon Ward-Fore, MS, MT(ASCP), CIC: “Practices drift. You can become complacent and maybe your level of awareness has decreased…. So, infection preventionists need to be really aware of what’s happening in the areas they cover as far as PPE usage is concerned.”