
Sterile Processing
Latest News

Latest Videos

Shorts

More News

Thank you, IPC professionals, from Infection Control Today!

With hospitals across the country accelerating investments in automation, why does human competence remain vital?

We all know that preventing employee fatigue and burnout requires a multipronged and ongoing effort to address the issue. There’s probably not a company in this nation that hasn’t experienced exhausted and frustrated employees, and the struggle is real to keep everybody engaged and motivated.

Sherrie is back! In this The Clean Bite, she discusses single-use vs Resusable Dental Supplies. Ultrasonic scaler tips, burs, and endodontic files are often reused despite being marketed as single-use. How many patients are at risk from shortcuts in reprocessing?
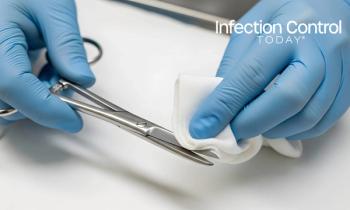
What is a day in the life of a sterile processing department technician like? Read this article by Hannah Schroeder, BSHA, CRCST, CHL, CIS, CER, to find out.

As ambulatory surgery centers (ASCs) expand into new specialties, sterile processing challenges can slow growth or halt operations entirely. Lifeline Surgical Partners—formerly Lifeline Vascular Care—found a scalable, cost-effective solution through offsite reprocessing, allowing their centers to maintain high-quality care while freeing clinical teams to focus on patients.

When traditional cleaning can’t reach hidden biofilm, ultrasonic cleaning steps in—delivering precision, safety, and efficiency across modern medical and dental care.

From ultrasound gel safety to high-level disinfection, The Joint Commission’s 2025 surveys are zeroing in on infection prevention hot spots. Are your teams ready?
Ensuring the sterility of medical devices is a cornerstone of patient safety. This whitepaper examines steam sterilization—the predominant method in healthcare—and the critical role of chemical indicators (CIs) in monitoring process efficacy. With a focus on ISO 11140-1 standards, it compares Type 4 and Type 5 indicators, outlining their strengths, limitations, and implications for reliable sterilization practices.

Check out the latest print edition of Infection Control Today: September/October 2025.
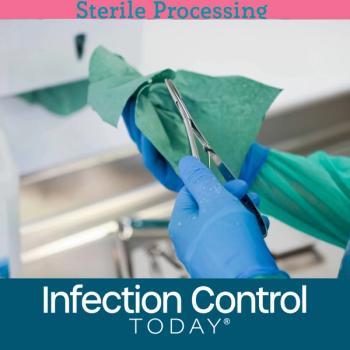
How can health care facilities and health providers implement point-of-use (POU) instrument care? Here’s a closer look at the entire process, the principles guiding its implementation, and the potential barriers to implementing point-of-use instrument care.

VIM-producing Pseudomonas aeruginosa isn’t just surviving in ICUs; it’s thriving. With mortality rates exceeding 30%, colonization risks hiding in drains, devices, and even donor milk, IPs must take proactive steps to outsmart this pathogen. Now is the time to double down on environmental controls, risk factor recognition, and surveillance strategies. Let’s break the biofilm cycle before the next outbreak takes root.

As climate change accelerates, health care’s environmental impact faces increased scrutiny, with sterile processing departments (SPDs) emerging as key change agents. Often behind the scenes, SPD professionals can lead sustainability by turning routine practices into ecofriendly protocols that protect both patient and planetary health.

Sharps safety isn’t just an operating room issue—it’s a system-wide concern that demands stronger policies, consistent reporting, and cross-departmental collaboration to truly protect health care workers.

Sterile processing departments are facing a new standard: clean is not clean unless you can see it. At HSPA 2025, experts emphasized that updated IFUs and borescope inspections must be built into routine workflows, not as extra tasks, but as core components of quality control and infection prevention.

Despite decades of progress in health care safety, a quiet but dangerous culture still lingers: many health care workers remain afraid to report sharps injuries, fearing blame more than the wound itself.

At the 2025 HSPA Annual Conference & Expo, Cori L. Ofstead, MSPH, highlighted critical flaws in manufacturers’ instructions for use (IFUs) for orthopedic and neurosurgical instruments. From contradictory directions to unrealistic cleaning expectations, these IFUs often fail under real-world conditions, jeopardizing both patient safety and sterile processing workflows.

In an era defined by digital transformation and post-pandemic urgency, telemedicine has evolved beyond virtual visits to become a vital infrastructure for delivering personal protective equipment (PPE) and managing sterile supplies. By enabling real-time forecasting, remote quality control, and equitable distribution, telemedicine is revolutionizing how health care systems protect both patients and providers.

Despite being a well-known occupational hazard, sharps injuries continue to occur in health care facilities and are often underreported, underestimated, and inadequately addressed. A recent interview with sharps safety advocate Amanda Heitman, BSN, RN, CNOR, a perioperative educational consultant, reveals why change is overdue and what new tools and guidance can help.

Despite their smooth, polished exteriors, surgical instruments often harbor dangerous contaminants deep inside their lumens. At the HSPA25 and APIC25 conferences, Cori L. Ofstead, MSPH, and her colleagues revealed why borescopes are an indispensable tool for sterile processing teams, offering the only reliable way to verify internal cleanliness and improve sterile processing effectiveness to prevent patient harm.

A groundbreaking study presented at HSPA25 and APIC25 exposed hidden contamination lurking inside orthopedic and neurosurgical instruments—even after cleaning. The Lumens 2.0 research highlights why infection prevention must look deeper than surface-level protocols.

In the heart of the hospital, decontamination technicians tackle one of health care’s dirtiest—and most vital—jobs. At HSPA 2025, 6 packed workshops led by experts Jill Holdsworth and Katie Belski spotlighted the crucial, often-overlooked art of PPE removal. The message was clear: proper doffing saves lives, starting with your own.

As measles cases climb across the US, discredited myths continue to undercut public trust in vaccines. In an exclusive interview with Infection Control Today, Michigan Medicine’s Marschall Runge, PhD, confronts misinformation head-on and explores how clinicians can counter it with science, empathy, and community engagement.

In this interview, completed shortly before the HSPA 2025 conference, as she prepared to take the helm as HSPA’s next president, Arlene Bush, CRCST, CER, CIS, SME, DSMD, CRMST, discusses humility, determination, and a bold vision to elevate sterile processing professionals and broaden the association’s impact.

At the 2025 Healthcare Sterile Processing Association (HSPA) Annual Conference & Expo, Cheron Rojo, BS, FCS, CHL, CIS, CER, CFER, CRCST, spotlighted real-world gaps in sterile processing education, stressing the urgent need for better tools, training, and collaboration when handling intricate medical devices like shaver handpieces.










