
Sterile processing of robotic surgery instruments and other complex instruments require complex methods.

Sterile processing of robotic surgery instruments and other complex instruments require complex methods.

Take 5 minutes to catch up on Infection Control Today’s highlights for the week ending May 6.
The surface material and product damage caused by new advances in disinfection technology must be addressed for patient safety.

Take 5 minutes to catch up on Infection Control Today’s highlights for the week ending April 29.

Terra Kremer, the senior program manager of microbiological quality and sterility assurance at Johnson & Johnson, and technical lead of the Device Processing Tiger Team, spoke with ICT® about her research on time and efficiency of sterile processing and what is most affecting the industry today.

The HSPA 2022 presentation focused on sharing new and future sterilization standards updates.
Total dissolved solids, pH levels, and iron levels are only a few components that must be considered.

Jill Holdsworth, CIC, FAPIC, NREMT, CRCST, manager of infection prevention at Emory University Hospital Midtown, joins ICT® to discuss the implications of her research, as well as other guidance for building an optimal relationship between infection prevention and the sterile processing department.

It starts with always asking “why” and with volunteering to research “the way it’s always been done."
Halting transmission of infectious pathogens in the sterile processing department hinges on proper personal protective equipment, proper hand hygiene, and proper cough and sneeze etiquette.

Take 5 minutes to catch up on Infection Control Today’s highlights for the week ending April 15.

Susan “Suzy” Scott, MSN, RN, WOC Nurse, also speaks to Infection Control Today® about incident tracking and electronic medical records.

After an Expo like no other, Dennis looks forward to the 70th year of advocacy for perioperative nurses.

Take 5 minutes to catch up on Infection Control Today’s highlights for the week ending April 8.
From 2014 to 2021, continued FDA reports have shown multidrug-resistant bacteria contaminating 6 types of endoscopes have had fatal consequences.

The letter comes after a year of reprocessing validation testing and a voluntary medical device recall.
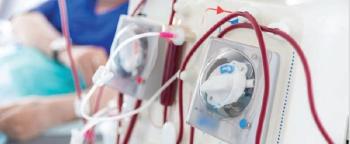
The hemodialysis setting presents a challenge for environmental cleaning and disinfection because of the demand for rapid turnover of stations.

Summary: Take 5 minutes to catch up on Infection Control Today’s highlights for the week ending March 18.
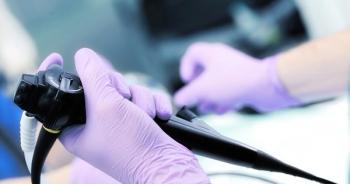
ANSI/AAMI’s update on endoscope processing is backed by rigorous scientific evidence.

Take 5 minutes to catch up on Infection Control Today’s highlights for the week ending March 11.
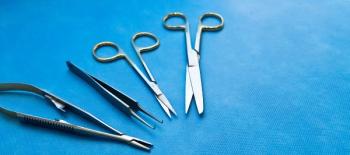
A letter has been sent to more than 4500 veterans stating that reusable instruments used in medical procedures may not have been sterilized properly at a Georgia hospital.

Take 5 minutes to catch up on Infection Control Today’s highlights for the week ending March 4.
Staff shortages, public recognition, and moving out of the medical field are all issues that the sterile processing industry has faced.
The ability to be an excellent infection preventionist requires lifelong learning and taking the initiative to grow professionally.
The successful combination of products and the adoption and application of science-based practices will help the sterile processing profession rise above challenges to protecting frontline technicians.