Sterile Processing
Latest News















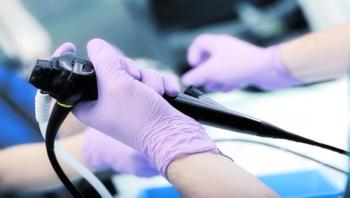
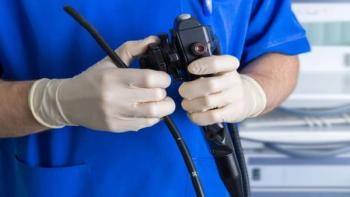
The reprocessing procedure for flexible endoscopes is an intricate multi-step process that requires a significant amount of time and diligence that can vary by endoscope type and manufacturer.




Q: We reprocess vaginal specs for some local doctors' offices. I recently learned that once the office staff receives the instruments, they are then opening up the individual sterile peel pouches and then place the “unprotected” vaginal speculums into the exam table to be ready for use by the provider. Is there anything we can purchase to use for transportation purposes that will save us time and money and skip the sterilization process of these items?A: This is an excellent question, especially with many sterile processing departments (SPDs) now processing devices for offsite clinics and doctors' offices. Vaginal speculums fall under the semi-critical devices category (Spaulding).

ICT spoke with Karen Swanson LPN, CSPM, CFER, manager of the central sterile department at Connecticut Children's Medical Center and chairman of the board of directors of the Certification Board for Sterile Processing (CBSPD), regarding the challenges that face sterile processing professionals and the importance of building key skill sets.