
Sterile Processing
Latest News




AAMI Foundation Publishes Infusion Therapy Safety Quick Guide

Sanovas Launches Infection Control Business to Address Expanding Threat of HAIs

Protein Disrupts Infectious Biofilms
Many infectious pathogens are difficult to treat because they develop into biofilms, layers of metabolically active but slowly growing bacteria embedded in a protective layer of slime, which are inherently more resistant to antibiotics. Now, a group of researchers at Caltech and the University of Oxford have made progress in the fight against biofilms. Led by Dianne Newman, the Gordon M. Binder/Amgen Professor of Biology and Geobiology, the group identified a protein that degrades and inhibits biofilms of Pseudomonas aeruginosa, the primary pathogen in cystic fibrosis (CF) infections.

FDA Releases Recommendations to Combat Cross-Contamination from Endoscopes

US Endoscopy Announces Full Market Release of BioGuard Air/Water and Suction Valves

TSO3 Receives 2017 Purchase Orders from Getinge

FDA Updates Information on Automated Endoscope Reprocessors

Optim LLC Releases Rapicide Peracetic Acid Compatible ENTityXL Nasopharyngoscope

Molecular Chameleons Reveal Bacterial Biofilm

Best Practices for Transporting Soiled Items

Innovative Health Enters Into an Agreement With Intalere for Reprocessing Services

Xenoscope Receives CE Mark


Heater-Cooler Devices Blamed for Global Mycobacterium chimaera Outbreak

ECRI Institute's Annual Health Technology Hazards List Includes Infection Risks

CS Medical Announces FDA Clearance of Single-Use, High-Level Disinfectant Chemistry

Divergent Views Expressed at FDA's Third-Party Service Workshop

Creating a Slippery Slope on the Surface of Medical Implants


FDA Conducts Inquiry of Third-Party Servicing and Refurbishment of Medical Devices
To help address the tough questions related to its inquiry into third-party servicing and refurbishment of medical devices, the Food and Drug Administration (FDA) scheduled a two-day public workshop for late October. Stakeholders are many and quite diverse, ranging from clinicians, patient safety managers and HTM professionals, to original equipment manufacturers (OEMs) and those aforementioned third-party firms; all of whom will be providing their perspectives on how to ensure patient safety while controlling costs in an environment where organizations vary in how they repair and generally maintain devices.

FDA Releases Draft Guidance on the Evaluation of Software as a Medical Device
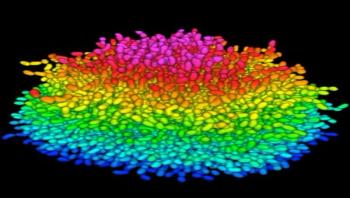
Understanding Bacteria's Slimy Fortresses
Princeton researchers have for the first time revealed the mechanics of how bacteria build up slimy masses, called biofilms, cell by cell. When encased in biofilms in the human body, bacteria are a thousand times less susceptible to antibiotics, making certain infections, such as pneumonia, difficult to treat and potentially lethal.
