
Sterile Processing
Latest News











At the two hospitals where I work, the sterile processing departments (SPDs) are located in the basement and near the morgue. To think that people are literally dying to be closer to the SPD is a unique feature that not just any hospital department can claim!

Q: Are we allowed to use latex gloves in the decontamination area? Is there any documentation in AAMI or OSHA that dictates the use of latex gloves in the decontamination area? Secondly, are we allowed to wear gloves in the sterile prep area while putting together trays?A: According to the Occupational Safety & Health Administrations’ Blood Borne Pathogen Ruling (2001), “Personal protective equipment (i) Provision. When there is occupational exposure, the employer shall provide, at no cost to the employee, appropriate personal protective equipment such as, but not limited to, gloves, gowns, laboratory coats, face shields or masks and eye protection, and mouthpieces, resuscitation bags, pocket masks, or other ventilation devices. Personal protective equipment will be considered “appropriate” only if it does not permit blood or other potentially infectious materials to pass through or reach the employee’s work clothes, street clothes, undergarments, skin, eyes, mouth, or other mucous membranes under normal conditions of use and for the duration of time that the protective equipment will be used. Accessibility. The employer shall ensure that appropriate personal protective equipment in the appropriate sizes is readily accessible at the work site or is issued to employees. Hypoallergenic gloves, glove liners, powderless gloves, or other similar alternatives shall be readily accessible to those employees who are allergic to the gloves normally provided.”

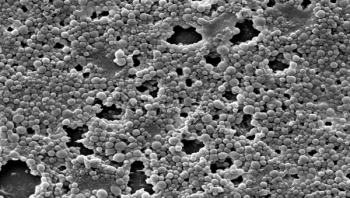
Biofilms, or colonies of bacteria growing on surfaces and medical devices, can inflict intractable or recurring disease. During colonization, biofilms develop characteristics and behaviors more dangerous and powerful than those of planktonic (singleton) bacteria. In fact, these insidious microscopic collectives could be regarded as biological case studies in “strength in numbers” as they unify against external assault, resisting the host immune response as well as antimicrobials, and exact their high human and fiscal costs. Puzzlingly, although biofilms are a ubiquitous, well documented cause of infection, they receive only a modicum of the attention they clearly merit.




Learning how to use new healthcare technology is a complex challenge, and success hinges on high-level support at any organization, according to nurses, vendors, and other experts who attended the AAMI Foundation’s first Industry Council meeting. This diverse group-which included representatives from BD (formerly CareFusion), Connexall, Hospira (a Pfizer company), Masimo, and Medtronic, as well as patient safety advocates and healthcare professionals-met at AAMI’s headquarters in Arlington, Va. recently, to discuss the current state of training, identify challenges, and describe what they would like to see in the future.




Q: Recently our facility was cited for disinfected laryngoscope blades that were found unprotected from re-contamination in storage. What is the recommended practice for these items?A: This question has many implications. CDC’s Healthcare Infection Control Practices Advisory Committee (HICPAC) says laryngoscope blades are “semicritical” items, which are defined as, “Items that directly or indirectly contact mucous membranes of the respiratory tract. They should be sterilized or subjected to high-level disinfection before reuse.” After they are cleaned according to the manufacturer’s IFU, there are several options for processing laryngoscope handles and blades. Many laryngoscope blades can be high-level-disinfected. If high-level disinfection is used (check the manufacturer’s IFU for compatible chemicals), the blade must be protected from recontamination after processing. One way of accomplishing this is to place the blade in a zip-lock bag and then apply a “Clean Not Sterile” label to the top of the bag. (Make sure that you clean your hands first.) If anyone opens the bag, the label will be damaged indicating the blade could be contaminated. At some facilities, laryngoscope blades are sterilized, which is acceptable but not necessary (CDC, 2003). Packaging blades requires the package to be opened if nursing must test the laryngoscope bulb on the blade. This results in a blade being replaced inside an open paper-plastic pouch. The opened pouch does not protect the blade from contaminates.


