
Sterile Processing
Latest News



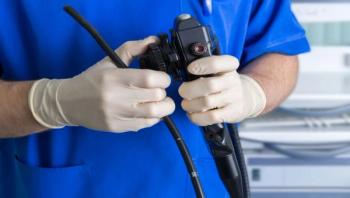
In mid-May, the Food and Drug Administration (FDA) convened a special meeting of the Gastroenterology-Urology Devices Panel of the Medical Devices Advisory Committee as a continuance of its examination of end-user challenges associated with reprocessing endoscopes including duodenoscopes. In previous communications, the FDA has expressed its concerns regarding the risks to patients if flexible endoscopes and their accessories are not cleaned properly and reprocessing guidelines not followed. The agency has also held several stakeholders' meetings and workshops, including with the Association for the Advancement of Medical Instrumentation (AAMI) that focused on factors affecting reprocessing of reusable medical devices and established clarion themes that began to outline key challenges and priority actions. In addition, in mid-March, the FDA issued its Final Guidance that addressed mandates for manufacturers relating to validation methods and labeling of medical devices that are reprocessed.

The Association for periOperative Registered Nurses (AORN) is currently updating its guideline on flexible endoscope reprocessing. ICT spoke with Sharon Van Wicklin, MSN, RN, CNOR, CRNFA(E) CPSN-R, PLNC, a perioperative nursing specialist for AORN who is responsible for this undertaking. She shares her thoughts about the opportunities and challenges associated with revising a guideline of this magnitude.


Q: Recently, we ran out of the single-use filters for our rigid containers. I learned the staff was making their own filters out of packaging material (see photo below). What advice can you provide when something like this happens?

May 2015 marked my 18th month of working as a sterile processing technician. A lot has happened during the past year and a half, so this is a good time to reflect on what I have learned about this important but behind-the-scenes profession in the healthcare industry.







Q: I was recently informed we will be performing surgery on a patient with suspect Creutzfeldt-Jakob Disease (CJD). We were informed that all the instruments should be soaked in bleach or discarded after the surgery. Can you provide some current recommendations on this?A: There has been much attention to CJD over the years. CJD is a rare, fatal neurological disease cause by an infectious protein, hence the term “prion.” The prions are very resistant to all forms of inactivation including regular steam cycles, ETO, gas and vapor phase peroxide, etc. To add to the confusion, there are recommendations from the World Health Organization (WHO), Centers for Disease Control and Prevention (CDC) and the Association for the Advancement of Medical Instrumentation (AAMI) ST-79, Annex C. Some of the recommendations from these different organizations conflict with one another.





Life science companies continually evaluate their operations for opportunities to improve throughput, increase compliance, and reduce costs. Medical device manufacturers, laboratories, and R&D facilities can all experience greater flexibility and more control with an in-house ethylene oxide (EO) sterilization program. By reducing what can be a two-week turnaround time with outsourced sterilization, operations managers can expect faster release of finished goods and increased inventory turns for a measurable and sustainable economic benefit. In particular, companies that process small, high-value devices can benefit from the new 3M™ Steri-Vac™ Sterilizer/Aerator GSX Series ethylene oxide (EO) sterilization system, with state-of-the-art mechanical design, real-time cycle information on the high-resolution color touch screen, and the ability to quickly and easily customize sterilization cycles.




