
Sterile Processing
Latest News


Biofilms -- formed by bacteria that stick to each other on living tissue and medical instruments, making them harder to remove -- can be tricked into dispersing with the targeted application of nanoparticles and heat, researchers have found. The University of New South Wales study, jointly led by associate professor Cyrille Boyer of the School of Chemical Engineering and deputy director of Australian Centre for NanoMedicine, appears in today's issue of Nature's open access journal Scientific Reports.










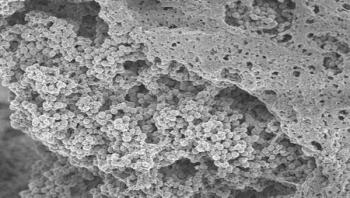
Biofilms frequently coat the surfaces of catheters, and of various medical implants and prostheses, where they can cause life-threatening infections. New research at the Sahlgrenska Academy show that coating implants with a certain "activator" can prevent Staphylococcus aureus, the leading cause of hospital-acquired infections, from forming biofilms.





Under perfect circumstances, surgical instruments are carefully handled, transported and cleaned-according to industry best practices and OEM instructions. But in a busy surgery department or facility, the rush of procedures and pressure to turn equipment around quickly can lead to shortcuts that ultimately damage surgical instruments.
Complacency in the high-level disinfection (HLD) and manual pre-cleaning of endoscopes is never an option. In order to ensure competency, an institutional quality program with written policies and procedures for endoscope processing must be established and strictly followed. These policies should be based on the Society of Gastrointestinal Nurses and Associates (SGNA) and the American Society of Gastrointestinal Endoscopists (ASGE) guidelines for the reprocessing of endoscopes.


Healthcare technology management (HTM) professionals and device manufacturers created a roadmap for solving issues of supportability during the AAMI Forum on Supportability of Healthcare Technology, held Nov. 2-3, 2015. More than 30 stakeholders representing HTM, industry, regulatory bodies, academic institutions, and others attended the interactive meeting, presenting their supportability concerns and working toward a framework for developing solutions. More than 30 stakeholders representing HTM, industry, regulatory bodies, academic institutions, and others attended the interactive meeting, presenting their supportability concerns and working toward a framework for developing solutions.


