
Sterile Processing
Latest News










In October 2011, more than 275 participants convened at the Food and Drug Administration (FDA) headquarters in Silver Spring, Md., for a multidisciplinary Medical Device Reprocessing Summit sponsored by the FDA in collaboration with the Association for the Advancement of Medical Instrumentation (AAMI). The fall 2011 summit built on an FDA public workshop on reprocessing in summer 2011. For all participants, the summit proved to be an opportunity for a renewed emphasis on performing all the necessary steps in reprocessing reusable medical devices to ensure clean and disinfected or sterilized devicesnot just in the universe of regulations, standards, and best practices, but also in the harried clinical environments and diverse sterile processing centers that are ground zero for reprocessing.

The International Association of Healthcare Central Service Materiel Management (IAHCSMM) has long been an outspoken advocate of state certification of central service (CS) professionals. Certification and continuing education credits keep technicians up-to-date on standards-based instrument processing practices, so these professionals can be skilled, competent and confident in their ability to keep patient safety and quality at the forefront.









Virginia Tech scientists have provided new evidence that biofilms bacteria that adhere to surfaces and build protective coatings are at work in the survival of the human pathogen Salmonella.
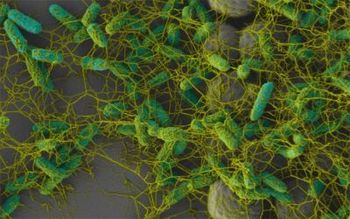
New research from Harvard University helps to explain how waterborne bacteria can colonize rough surfaceseven those that have been designed to resist water.



